Media
HKU Biologists Reveal a Novel Macrophages-mediated Mechanism that Promotes Peritoneal Metastasis of Ovarian Cancer,
Providing Important Insights into Its Therapeutic Strategy
16 May 2022
A research team at the School of Biological Sciences, the University of Hong Kong (HKU), has revealed novel cellular and molecular interactions between cancer cells and tumour-associated macrophages that promote peritoneal metastasis of ovarian cancer. These findings provide important insights into the therapeutic strategy of ovarian cancer and are now published in Advanced Science, a leading interdisciplinary open-access journal.
Background
Ovarian cancer is the leading cause of deaths among all gynaecological cancers. Over 70% of patients are diagnosed at an advanced stage with metastatic diseases. Peritoneal metastasis is very difficult to treat due to tumour heterogeneity and the dynamic interactions of cancer cells with the tumour microenvironment. The lack of suitable experimental models has been one significant obstacle to study the cellular and molecular mechanisms of this critical process, and the distinct interactions among different cancer cell subclones and tumour microenvironment are largely unknown using traditional bulk measurement.
Research methods and findings
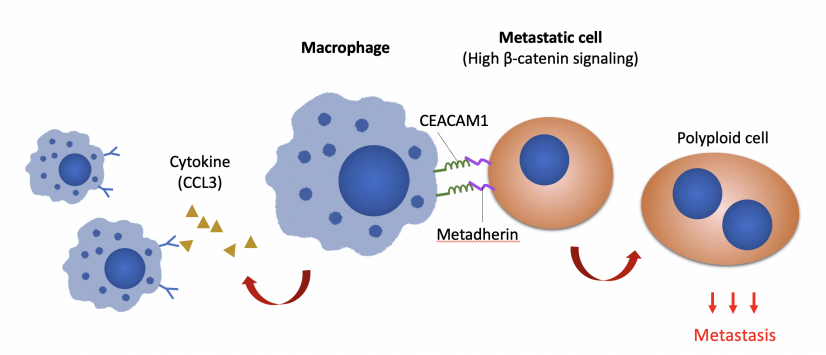
Key findings: In metastatic ovarian cancer cells, Wnt/b-catenin signalling upregulates the expression of metadherin, which communicates with macrophages through CEACAM1, a carcinoembryonic antigen expressed by macrophages, suggesting that blockade of macrophage-tumour communications (by inhibiting either metadherin or CEACAM1) could greatly reduce peritoneal metastasis.
Based on tumour heterogeneity, the research team has previously established an isogenic model that mimics spontaneous ovarian cancer metastasis. Using this model, an upregulation of Wnt/b-catenin signalling was found in the metastatic cells by gene profiling and bioinformatic analyses. Wnt/b-catenin signalling is known to play critical roles in embryonic development, tissue homeostasis and cancer development, since its upregulation increases oncogene expression and facilitates cancer metastasis.
Macrophages play key roles in both the innate and adaptive immunity to orchestrate the concerted immune responses and are the most abundant immune cells in the ovarian cancer tumour microenvironment. Observation of cellular behaviours using single-cell time-lapse microscopy reveals that in the presence of macrophages, a subset of the metastatic cells shows selective advantage of becoming polyploidy, a phenotype that duplicates entire genomes, could promote tumour aggressiveness and therapeutic resistance. On the other hand, the metastatic cells polarise macrophages to a tumour-associated phenotype that reinforces the polyploid phenotype. Further molecular analyses suggest that b-catenin signalling upregulates cancer cell surface metadherin, which communicates through CEACAM1 expressed by macrophages.
The clinical relevance of these scientific findings were further validated by tumour xenografts in mice and patients’ clinical samples. Blocking macrophage-tumour communications via the inhibition of metadherin or CEACAM1 greatly reduced peritoneal metastasis in humanised mouse models which have human immune cells. Since metadherin and CEACAM1 are accessible on the outer surface of cells, they represent highly suitable candidates for tumour cell tracking and clinical targeting.
Research significance
The team has made a key discovery of a potential driving mechanism for cancer cell polyploidy and genomic instability, which is initiated through direct interaction with macrophages. Targeting components of the molecular cascade identified in the study holds great therapeutic potential to disrupt polyploidisation of the cancer subclones that drive metastasis.
‘Our findings are intriguing because few factors that regulate cancer polyploidy have been identified to date, and we have also provided a mechanistic rationale for targeting b-catenin or its downstream signalling molecules to decrease peritoneal dissemination associated with poor prognosis,’said Professor Alice WONG, Director (Interim) of the School of Biological Sciences, who led the research. The team plans to explore in more detail the signalling mechanisms that drive polyploidy in the metastatic cells, as this would greatly enhance the understanding of the genomically unstable disease.
About the research team
The research was co-led by Professor Alice Sze Tsai WONG (Director (Interim) of School of Biological Sciences, HKU), and Dr Jue SHI (Associate Professor, Department of Physics, Hong Kong Baptist University (HKBU)). Dr Sally Kit Yan TO (Postdoctoral Fellow, School of Biological Sciences, HKU), was the first author, with the assistance of Dr Maggie Kei Shuen TANG (Postdoctoral Fellow, Laboratory for Synthetic Chemistry and Chemical Biology, InnoHK; Honorary Research Associate, School of Biological Sciences, HKU) and Dr Yin TONG (Postdoctoral Fellow, Department of Pathology, HKU). Other collaborators include Dr Jiangwen ZHANG (Associate Professor, School of Biological Sciences, HKU), Dr Karen Kar Loen CHAN, Clinical Associate Professor, Department of Obstetrics and Gynecology, HKU), and Dr Philip Pun Ching IP (Clinical Associate Professor, Department of Pathology, HKU).
Acknowledgements
This work was supported by the Hong Kong Research Grant Council grants (17104820, 17141216, C4041-17G and C2006-17E) and ‘Laboratory for Synthetic Chemistry and Chemical Biology’ under the Health@InnoHK Program launched by Innovation and Technology Commission, HKSAR. Professor Alice WONG is a recipient of the Croucher Foundation Senior Research Fellowship.
Link of journal paper can be accessed from here: https://doi.org/10.1002/advs.202103230
More information about Professor Alice WONG’s work and her research team: https://www.awonglab.com/
Image download and caption: https://www.scifac.hku.hk/press
For media enquiries, please contact Ms Casey To, External Relations Officer (tel: (852)3917-4948; email: caseyto@hku.hk) / Ms Cindy Chan, Assistant Director of Communications of HKU Faculty of Science (tel: (852)3917-5286; email: cindycst@hku.hk).